24 june 2017.
Learning Basics physics.
In 1924, Louis-Victor de Broglie formulated the de Broglie hypothesis, claiming that all matter,[15][16] not just light, has a wave-like nature; he related wavelength (denoted as λ), and momentum (denoted as p):
 and the wavelength (in a vacuum) by λ =
and the wavelength (in a vacuum) by λ =  , where c is the speed of light in vacuum.
, where c is the speed of light in vacuum.
De Broglie's formula was confirmed three years later for electrons (which differ from photons in having a rest mass) with the observation of electron diffraction in two independent experiments. At the University of Aberdeen, George Paget Thomson passed a beam of electrons through a thin metal film and observed the predicted interference patterns. At Bell Labs, Clinton Joseph Davisson and Lester Halbert Germer guided their beam through a crystalline grid.
De Broglie was awarded the Nobel Prize for Physics in 1929 for his hypothesis. Thomson and Davisson shared the Nobel Prize for Physics in 1937 for their experimental work.
Learning Basics physics.
De Broglie's wavelengthEdit

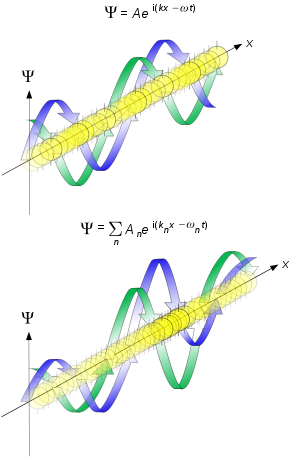
Propagation of de Broglie waves in 1d—real part of the complex amplitude is blue, imaginary part is green. The probability (shown as the colour opacity) of finding the particle at a given point x is spread out like a waveform; there is no definite position of the particle. As the amplitude increases above zero the curvature decreases, so the amplitude decreases again, and vice versa—the result is an alternating amplitude: a wave. Top: Plane wave. Bottom: Wave packet.
 and the wavelength (in a vacuum) by λ =
and the wavelength (in a vacuum) by λ =  , where c is the speed of light in vacuum.
, where c is the speed of light in vacuum.De Broglie's formula was confirmed three years later for electrons (which differ from photons in having a rest mass) with the observation of electron diffraction in two independent experiments. At the University of Aberdeen, George Paget Thomson passed a beam of electrons through a thin metal film and observed the predicted interference patterns. At Bell Labs, Clinton Joseph Davisson and Lester Halbert Germer guided their beam through a crystalline grid.
De Broglie was awarded the Nobel Prize for Physics in 1929 for his hypothesis. Thomson and Davisson shared the Nobel Prize for Physics in 1937 for their experimental work.

In 1924, Louis-Victor de Broglie formulated the de Broglie hypothesis, claiming that all matter,[15][16] not just light, has a wave-like nature; he related wavelength (denoted as λ), and momentum (denoted as p):
ReplyDelete\lambda ={\frac {h}{p}}